EMA finds ‘possible link’ between AstraZeneca vaccine and blood clots
- 07-04-2021
- Business
- Canarian Weekly
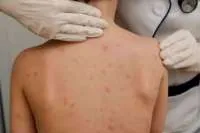
Europe’s medicines regulator, the EMA, have announced a ‘possible link’ this afternoon (Wednesday) between the coronavirus vaccine developed by AstraZeneca and the University of Oxford, and rare blood clotting issues in adults who received the shot, and that packaging should carry a warning of the rare side effects. It comes after a review of all currently available evidence into extremely rare cases of unusual blood clots in some vaccinated people.
Emer Cooke, executive director of the European Medicines Agency, said in a press conference that the regulator’s safety committee “has confirmed that the benefits of the AstraZeneca vaccine in preventing Covid-19 overall outweigh the risks of side effects.”
The EMA’s safety committee “after a very in-depth analysis has concluded that the reported cases of unusual blood clotting following vaccination with the AstraZeneca vaccine should be listed as possible rare side effects of the vaccine,” said Cooke. “A plausible explanation for these rare side effects is an immune response to the vaccine similar to one seen in patients treated with heparin,”, noting that it’s called heparin-induced thrombocytopenia.
Other articles that may interest you...
Trending
Most Read Articles
Featured Videos
TributoFest: Michael Buble promo 14.02.2026
- 30-01-2026
TEAs 2025 Highlights
- 17-11-2025