Good news for Spain: The EMA approves the Moderna vaccine
- 07-01-2021
- Business
- Canarian Weekly
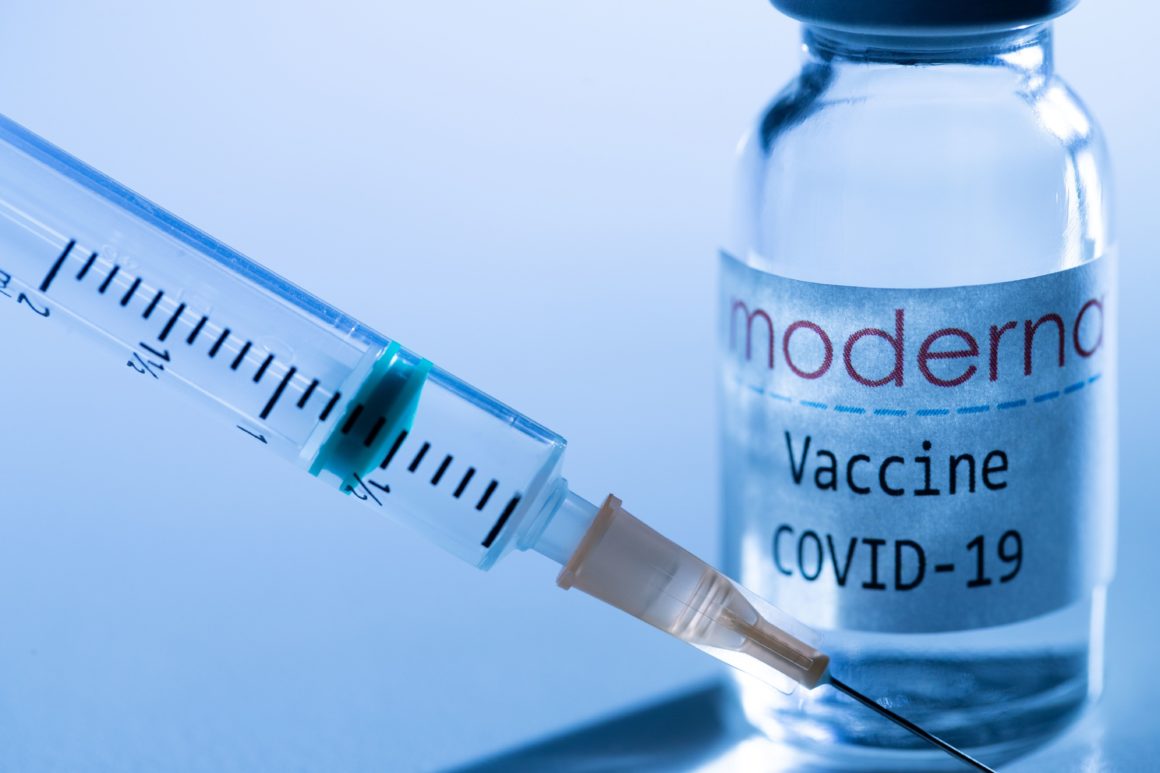
The European Medicines Agency (EMA) has given the green light to the commercialization in the EU of the Moderna vaccine which has been developed in Spain by BioTech. Nevertheless, after its examination, the EMA only recommends using this inoculation for those over 18 years of age, following the recommendations of the pharmacist itself, in its trials, it certified the efficacy of its formula from the age of 16.
The Ministry of Health says this will give a "strong boost" to the vaccination campaign against Covid with the arrival of the first vials of this second formula, which will be added to Pfizer products, the only ones so far being distributed in the European Union.
After the controversy over the short supply during the first week, the department headed by Salvador Illa hopes to significantly increase the rate with the incorporation of mRNA-1273, Moderna's formula that is almost identical in effectiveness to that of Pfizer, with a percentage close to 95% of immunization.
The EU are expected to give their approval today following the EMA’s thumbs up so that Moderna can start distributing as many vials of the vaccine as soon as possible to the member states.
Ursula von der Leyen, president of the European Commission, said minutes after hearing the EMA's decision; “We are working at full speed to approve it and make it available to the European Union." The laboratory's commitment is that within a period of fifteen days after the approval of the EMA, the first doses should be delivered said the Ministry of Health earlier this week.
The EU has purchased a total of 160 million vials of mRNA-1273, divided into two batches. As in the case of Pfizer, Spain is assigned by its population approximately one tenth of these, that is, about 16 million doses that, will serve to immunize 8 million ipeople. For Moderna, the booster dose should be administered one month after the first.
Health technicians believe that the arrival, delivery and inoculation of Moderna's vaccines will be much easier than that of Pfizer, not only because of the experience already acquired, but also because mRNA-1273 allows the transport and storage of the vaccine in its normal state, liquid at a temperature between two degrees positive and eight negative degrees , so conventional freezers are enough for its preservation, an advantage over the Pfizer compound that requires freezers that reach 70 degrees below zero.
Other articles that may interest you...
Trending
Most Read Articles
Featured Videos
TributoFest: Michael Buble promo 14.02.2026
- 30-01-2026
TEAs 2025 Highlights
- 17-11-2025