Intranasal vaccination could put an end to the Covid pandemic
- 26-09-2022
- Health
- Canarian Weekly
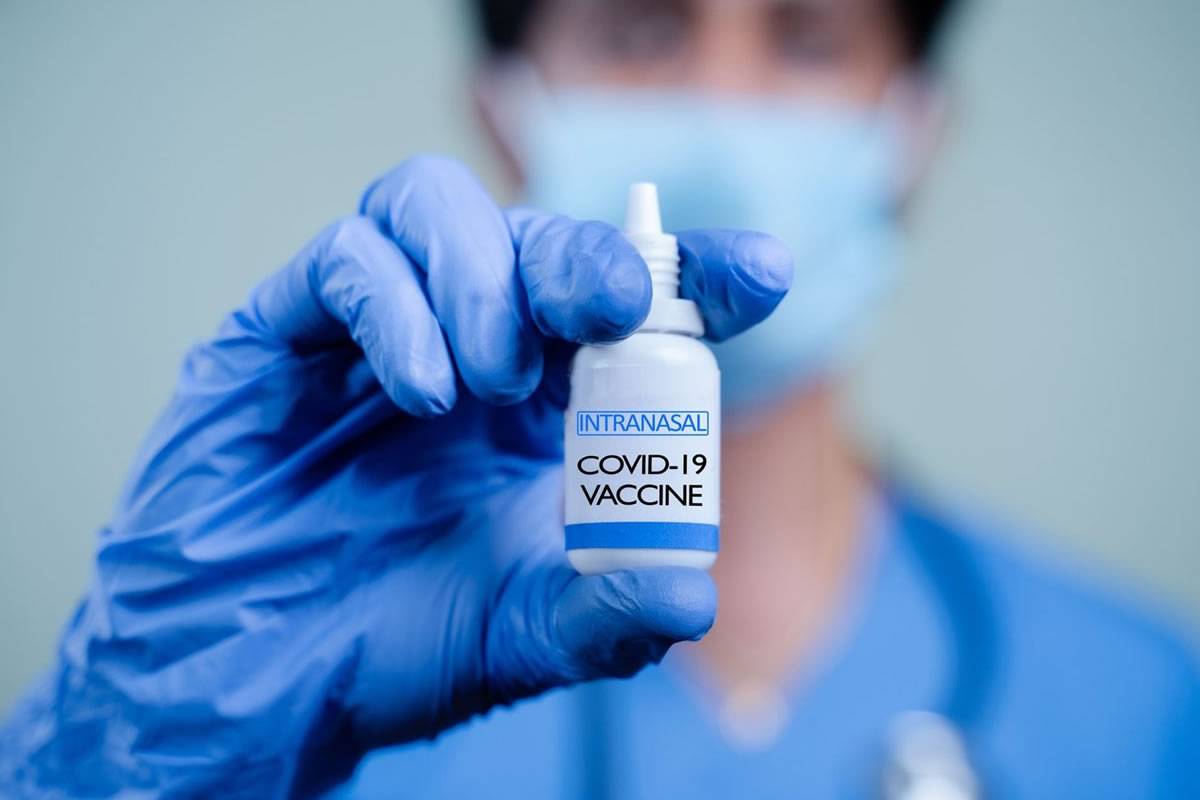
As the COVID-19 pandemic continues, new vaccines to prevent infection with SARS-CoV-2, the virus that causes COVID-19m are under constant development. Up to now, all approved Covid vaccines have been subcutaneous, that is, administered by injection into the upper area of the arm but now two companies are planning to change that through the development of COVID-19 vaccines inhaled through the nose.
CanSino Biologics, a Chinese pharmaceutical company, recently received approval from the National Medical Products Administration of China for Convidecia Air, their vaccine delivered via inhalation, as a nasal spray.
Bharat Biotech International, a company based in India, has also been granted approval, under Restricted Use in Emergency Situations in India, for its iNCOVACC vaccine administered intranasally as nasal drops.
What are nasal vaccines?
The most common way of administering a vaccine continues to be through an injection. However, this is not the first time scientists have developed an intranasal vaccine as the most widely known nasal vaccines right now are for the flu.
There have also been studies conducted on nasal vaccines for other diseases, including whooping cough, hepatitis B, and the African swine fever virus.
Some researchers believe that delivering a vaccine nasally provides the benefit of administering it directly into the mucosa of the body, which is the moist inner layer of body cavities, such as the nose and mouth, as well as some organs.
The mucosa is an important part of the body’s immune system. When a person breathes, the mucosa helps keep bacteria and other potentially problematic particles from getting into their body.
The mucosa also absorbs certain pathogens, and because the nose connects to the body’s respiratory system, this makes it easy for a nasal vaccine to move through the body.
Additionally, nasal vaccines provide less stress for people who are afraid of needles. Experts estimate that 25% of adults and 66% of children are afraid of having injections, and that 1 out of every 10 people may have put off having the Covid-19 vaccination due to their fear of needles.
COVID-19 nasal vaccines:
Both Convidecia Air from CanSino Biologics, and iNCOVACC from Bharat Biotech International Limited are recombinant vaccines, which means they use a protein from the SARS-CoV-2 virus in the vaccine.
When the vaccine enters the body, the protein attaches to cells in the body, teaching them to trigger an immune response if they ever encounter that same protein again.
Both of these intranasal vaccines also use adenovirus vector technology. Adenoviral vectors are genetically engineered viruses previously used in gene therapy.
According to statements on CanSino Biologics website, Convidecia Air uses the same adenovirus vector technological platform as Convidecia, the company’s injectable Covid-19 vaccine.
Convidecia recently received emergency use listing from the World Health Organization (WHO), after results from the phase 3 clinical trial showed a 57.5% efficacy rate against SARS-CoV-2 infection preventing symptomatic Covid, 28 days or more after vaccination.
Bharat Biotech’s iNCOVACC nasal vaccine was developed in partnership with Washington University St. Louis. The intranasal vaccine reportedly showed “successful results” following phase 1, 2, and 3 clinical trials.
The future of nasal COVID-19 vaccines:
Although these are the first two intranasal COVID-19 vaccines to receive approvals, there are others currently in development. For example, a team of microbiologists at Mount Sinai is currently developing one that is in, or has completed, phase 1 and 2 trials in Thailand, Brazil, Mexico, and Vietnam, with a phase 1 trial recently launched at Mount Sinai in the U.S.
In March, the University of Oxford reported the launch of phase 1 clinical trials investigating the delivery of a nasal COVID-19 vaccine it developed in partnership with AstraZeneca, and in May, Codagenix, a vaccine development company based in the U.S., announced the start of phase 1 clinical trials for its CoviLiv intranasal COVID-19 vaccine candidate.
Other articles that may interest you...
Trending
Most Read Articles
Featured Videos
TributoFest: Michael Buble promo 14.02.2026
- 30-01-2026
TEAs 2025 Highlights
- 17-11-2025